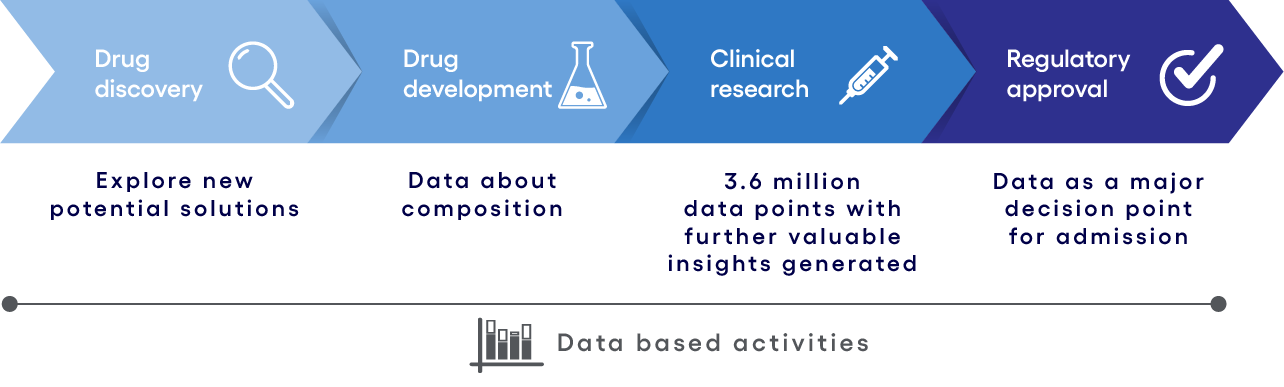
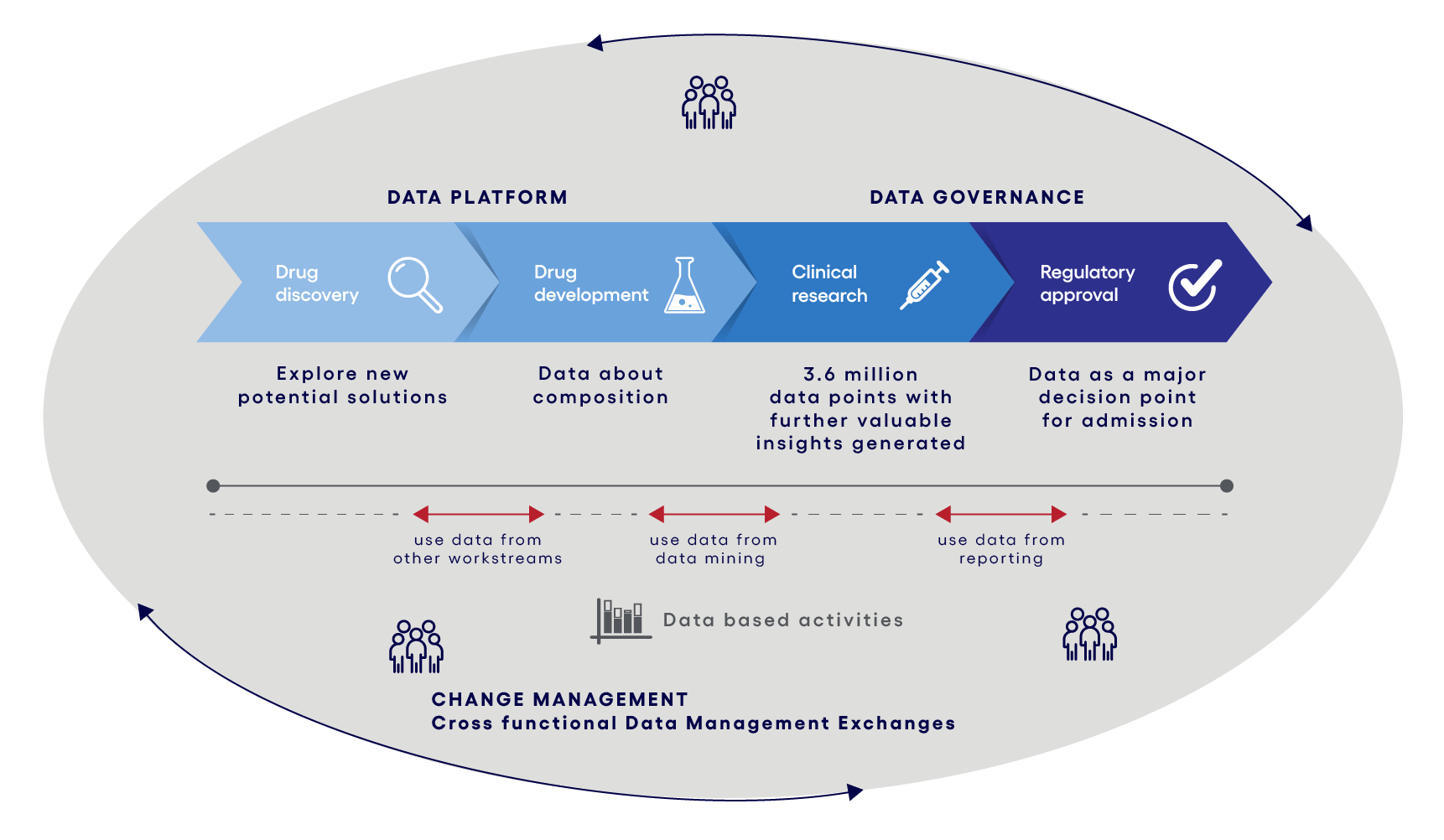
Figure 2: Data driven operating model
Outdated technologies leading to incorrect and insufficient data were identified as one reason for having data hidden in silos (Brown, 2020). Therefore, as a first step, a platform like Power BI, Tableau or Qlik with access to harmonized and updated data should be introduced. Cognizant Technology Solutions has been working on this topic for a long and can offer a broad range of experience. As an example, we have introduced a harmonized platform for clinical trial data at Roche in the year 2022. Such a new platform helps to ensure standardization and consistency of clinical trial data for study teams and other impacted stakeholders. With this a project could reduce their average questionnaire time by 36 %.
Such a newly generated platform needs to be supported by a proven governance structure. A governance structure provides guidance about who is having access to which data and ensures legal compliance. It is essential that those employees who need the data have access and can work with it.
For companies newly implementing such data bases it could be beneficial to involve data science experts to improve current low data competences. Additionally, those experts can accompany the introduction of data usage and data literacy within an organization.
After implementation of a data platform and governance structures, user adoption must be ensured. A study found out that only 20 % of the data and analytics solutions deliver business outcomes (Jain, 2022) due to lack of adoption.
To prevent this, it is highly recommended to work on a culture of data usage (especially beyond data analyzes) and data sharing across all workstreams. This can be achieved by leveraging experts in organizational change management.
A structured change strategy & plan supported by communication measures, tailored trainings, a change agent network and leadership alignment ensure a successful adoption across all workstreams. All new tools and ways of working need to be communicated within all available communication channels. R & D workers need to be trained how to use those new tools. A dedicated change agent network could support the interdisciplinary collaboration. Leadership alignment is essential, as the management must support the overall initiative and needs to provide a clear visual commitment for a data driven organization.
To ensure regular data exchange across all R & D functions communication channels such as SharePoint groups or regular exchange meetings could be established. Possible discussion topics within those communication channels could be the use of clinical trial data for further insights into the discovery of other drugs. The overall communication for example can be moderated and fostered by a dedicated and newly nominated data officer.
The overall goal of this is to adopt data sharing and data usage to everybody’s daily ways of working. Such new ways of increased working with data will lead to changes in the role profiles of the R & D workers. Therefore, Organizational Change Management (OCM) teams play a crucial part in assisting these changes. OCM helps to identify the job profile changes with a Change Impact Analysis. Based on that they are going to conduct communication measures and trainings to onboard all employees. It is important that all employees understand the benefits of the data structure. Goal is to make employees see the change as an upskilling of their role.
Cognizant has broad experience in extending use cases for clinical data. As an example, for one of our customers we have applied artificial intelligence techniques to clinical trial data to gain further insights regarding the optimal dosage for cancer drugs. This led to cost savings between 8% and 10 % per patient for our customer. In another project we developed a text mining algorithm to gain critical insights within 200’000 patient data. Those use cases proved the benefit of sharing data and using data beyond the already known ways of including data into our day-to-day work.